4.5 Factors Affecting Saturated Hydraulic Conductivity
As you may have expected, soil texture strongly influences saturated hydraulic conductivity. Soils dominated by large sand particles tend to have relatively large pore spaces and thus large values of saturated hydraulic conductivity. Soils dominated by small clay particles tend to have relatively small pores spaces and small values of saturated hydraulic conductivity. For example, using intact soil cores, Reynolds et al. measured saturated hydraulic conductivity values of 29 cm h-1 for a sand, 4.1 cm h-1 for a loam, and 0.091 cm -1 for a clay loam in Canada [5]. The saturated hydraulic conductivity of the sand was more than 300 times larger than that of the clay loam.
The presence, size, and continuity of macropores can also strongly influence saturated hydraulic conductivity. Poiseuille’s Law indicates that the flux through a tube increases with the square of the radius, and water flux through soil is also quite sensitive to the presence of large pores, even if they are few in number. Yet in the soil many other features of the pores are involved such as the connectivity, the internal roughness, and the tortuosity, which is a measure of the extent of twists and turns taken by the pores. Clayey soils with large numbers of well-connected macropores generated by living organisms in the soil, e.g. earthworms, can have saturated hydraulic conductivity values greater than those of coarse-textured soils which lack macropores [6].
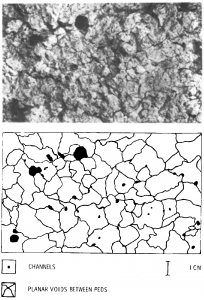
Likewise, the type and degree of soil structure also affect saturated hydraulic conductivity. Strongly developed, fine blocky soil structure contributes to high values of saturated hydraulic conductivity, while massive, featureless soil structure often indicates compaction and low values of saturated hydraulic conductivity [6]. By carefully measuring soil structural features such as the length, width, and number of inter-aggregate pores (Fig. 4‑5) and applying an appropriate model, one can reasonably predict the saturated hydraulic conductivity of a soil horizon [7]. In fact, some US states use visual inspection of the soil profile by trained soil scientists as a primary factor in determining the suitability of a location for the drain field of an on-site waste water treatment system, i.e. a septic system. The profile inspection allows the scientist to reasonably estimate the rate of water flow which the soil can sustain.
4.5.1 Chemical Dispersion and Flocculation
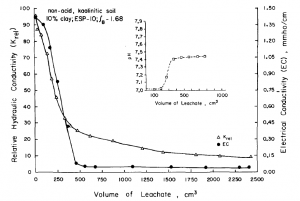
In addition to these physical properties, chemical properties of the soil and the solution flowing through the soil can also impact the saturated hydraulic conductivity. These chemical effects arise when soil and solution characteristics promote swelling and chemical dispersion of clay present in the soil. Chemical dispersion here is the process in which soil particles, which previously were held together in close contact within soil aggregates, respond to a changed chemical environment by expanding and separating from one another, breaking down the soil aggregates. Swelling and dispersion can reduce the saturated hydraulic conductivity of a soil by a factor of 100 or more [8]. Swelling and dispersion can be promoted by any of the following conditions: irrigation with sodic water [8]; a high content of 2:1 clays, particularly montmorillonite [9]; low electrical conductivity of the flowing solution [10]; or high exchangeable sodium percentage (ESP) in the soil. The ESP is defined based on the amount of exchangeable sodium in a soil divided by the sum of the exchangeable calcium, magnesium, potassium, and sodium. For example, leaching a column of sandy loam soil having an initial ESP of 10% with distilled water caused a 90% reduction in the Ks value (Fig. 4‑6).
Chemical dispersion of soil can sometimes be reversed, and the reverse process is called flocculation. Flocculation is the process in which dispersed soil particles come together, often due to a change in the chemical environment. A high proportion of polyvalent cations, such as Ca2+, Mg2+, and Al3+, promotes flocculation, while a high proportion of monovalent cations, particularly Na+, promotes chemical dispersion. Gypsum (CaSO4) has been successfully used to remediate chemical dispersion in sodic and saline-sodic soils because it provides Ca2+ to displace Na+ on the soil’s cation exchange sites [11]. Sulfuric acid (H2SO4) is another good remediation option for chemical dispersion in calcareous sodic soils because the acid dissolves calcium carbonate (CaCO3) present in the soil, and the Ca2+ released displaces Na+ [11].