Chapter 2: Principles of Aging and Biological Theories of Aging
Learning Objectives
By the end of this chapter, students should know, learn, or be able to do the following…
- Differentiate Gerontology and Geriatrics: Compare and contrast the fields of Gerontology and Geriatrics, elucidating their respective scopes, objectives, and contributions to the study of human development and aging processes across varying populations.
- Deconstruct Gerontological and Geriatric Dualisms: Evaluate and deconstruct prevalent dualisms within gerontological and geriatric science, analyzing their implications for theorizing, research, translation, and applied practice in aging and developmental science,
- Critique Biological Theories of Aging Categories: Analyze and critically appraise the two primary categories of biological theories of aging—Programmed and Random Error—by examining their underlying mechanisms, strengths, limitations, and implications for understanding the aging process.
- Synthesize Aging Principles from Biological and Genetic Perspectives: Synthesize the fundamental principles underpinning the aging process, integrating insights from biological and genetic perspectives, and assess how these principles contribute to the overall comprehension of aging mechanisms in biological development.
Chapter Outline
Gerontology and Geriatrics
There are two traditional disciplines focused on advancing the conceptualization, theoretical understanding, and empirical examination of successful aging in human development. These two disciplines include gerontology and geriatrics. Despite the fact that these two disciplines are commonly and interchangeably referenced as one in the same across many scientific domains and applied settings, each are independent disciplines which uniquely adhere to specific philosophical, conceptual, and theoretical viewpoints surrounding of human aging. Gerontology involves the multidisciplinary study of psychological, social, and cultural processes and functioning in old age. Many gerontologist traditionally adhere to a psychosocial model of aging, a theoretically approach emphasizing developmental change and stability in underlying social, emotional, and cognitive patterns of behavior, historical and recent patterns of continuity and discontinuity connected to one’s lived experiences and life-long aspirations, goals, and decision-making, and socio-cultural interactions and resources that allow aging individuals and populations to adapt and sustain a healthy standard of living and performance, as well as maintain positive quality-of-life throughout later adulthood and near the end-of-life.
Gerontologists are experts trained to implement holistic and human-centered services and programs designed and tailored for individuals and populations, aged 50 and older, across the second-half of life. Such experts translate theory into clinical social work and case management, deliver educational curriculum at institutions of higher learning as well as within adult and life-long learning programs, design age-friendly living environments, and implement and administrate local, state, and federal agencies and services across a variety of long-term and medical care, behavioral mental health, and social and community settings. Meanwhile, geriatrics entails the study of human genetics and biology relative to the diagnosis, treatment, and care individuals and populations challenged by acute and chronic disablement and disease in old age and near the end-of-life. Geriatrics is commonly grounded in the medical model, a theoretically-evolved perspective of aging as a health condition or disease that can be medically diagnosed, treated, and cured. Geriatricians are often recognized as medically specialized practitioners including but not limited to physicians, nurses, psychiatrists, physical and occupational therapists, and dieticians. Such experts are trained to provide specialized or skills-based medical care within the home health care, assisted-living, and long-term care industry, as well as in-patient and outpatient medical centers such as a hospital, urgent care clinic, or community health clinic.
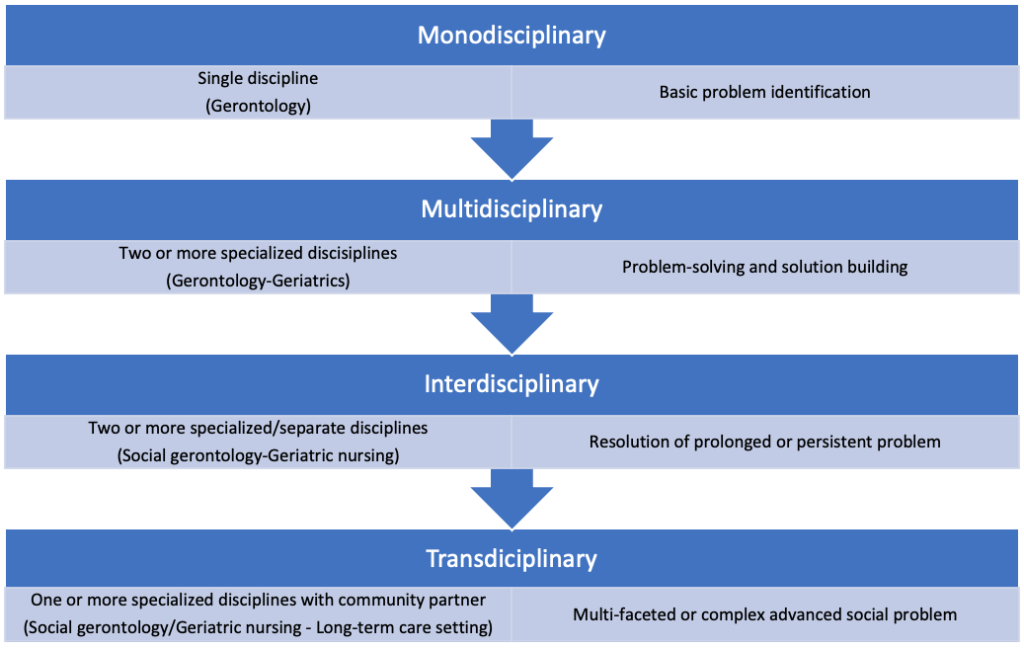
Although gerontology and geriatrics are independent and distinct scientific areas of inquiry, there is an interchangeable link between the two disciplines when it comes to the advancements in theorizing and theory-building on the topic of successful aging. This had entailed reliance on a four-tiered approach including (see Figure 2.1):
- Monodisciplinary or reliance upon one type or branch within a discipline in order to identify, define, and theorizing about a basic problem
- Multidisciplinary, or the practice of correspondingly borrowing and applying interchangeable theoretical conceptualizations from a variety of closely-related disciplines to address and solve a problem
- Interdisciplinary or the reliance and use of expertise from specialized disciplines to shift traditional theoretical models of understanding in ways that result in a conceptual redefinition or new theoretical approach of how to more accurately address or resolve a persistent problem and
- Transdisciplinary or mutual cross-blending of disciplinary thinking beyond academic specializations and into applied practice spaces usually involving community aging service providers or stakeholder partners seeking to solve complex social issues that may be too difficult to resolve without the cross-fertilization of academic and applied practice paradigms, concepts, or theory-based models.
Theoretical advancements across these four tiers has contributed to a contemporary blending of gerontology and geriatrics into a gerontology-geriatric link in theorizing and theory-building to understand how bioloical, psychological, and socio-cultural forces shape human aging and development. Two recent examples include the rise and development of comparative animal-human theoretical models to better understand genetic, behavioral, and environmental factors essential to supporting healthy and active aging (McCune & Promislow, 2021; Mitchell et al. 2015), as well as theoretical development surrounding human-machine learning to monitor and improve physical, cognitive, and social functioning and health of older adults (Speiser et al., 2021). The gerontology-geriatric collaboration in science is likely become more prominent within aging research and theory-building in order to achieve common ground and agreement surrounding how best to address theoretical dualities in human aging and development, as well as the advance and align paradigmatic thinking and theories with respect to contemporary biological, psychological and social states and traits essential for reducing vulnerability and increasing success in aging and development outcomes.
Theoretical Dualities of Studying Human Aging & Development
Nature vs. Nurture
Why do we age the way we do? Think about some of your physical features (e.g., height, weight, hearing/vision) and personal traits (e.g., emotion, personality, intellect). Do you ever ask yourself whether these features are a result of heredity or due to environmental factors; or perhaps a combination of both. You have likely observed the ways in which both heredity and environmental factors, such as lifestyle, diet, and other influences, have contributed to making you the way you are. For decades, developmentalists have continued to ask: Is aging and human development due to “nature or nurture.” Those who argue in favor of nature believe that genetics and heredity play the most essential role in human aging and development. Those who debate in support of nurture argue that environment provides the greatest influence in shaping the way we age and develop. The debate over the nature vs. nurture dualism will likely continue across aspects of inquiry into human development, yet most scholars tend to agree that there is a constant interplay between the two forces that seem to contribute significantly to the ways that human age and mature across time. Therefore, the root of any single human behavior or developmental outcome as a result solely due to nature or nurture remains inclusive.
Continuity vs. Discontinuity
Is human development best characterized as a slow and gradual process unfolding across time, or is it best viewed as one consisting of ages, stages, or developmental periods that bring about more abrupt change? The answer to that question often depends on which developmental theorist you might ask and what topic is being studied. Classical developmental theories such as those created by Freud, Erikson, and Piaget are referred to as stage theories, or the theoretical notion that human development and aging processes occur through universally distinct and sequential stages that are qualitatively different from one another. At each respective stage of development, humans express different qualities and characteristics, as well as fulfill certain age or period appropriate developmental tasks or milestones. In other words, stage theorists assume development is more discontinuous. However, developmental theories advanced by Vygotsky and information processing experts, coincide with continuous theories, or the assumption that human aging and development is a more slow and gradual process. For instance, such theorists would view adults as not possessing new skills; rather as person age over time they advanced skills and abilities that were already present in some form when they were a young child. Such experts would also contend that contextual and environmental experiences contribute to the acquisition, advancement, or adaptation of skills and abilities necessary for maturation.
Active vs. Passive
How much do you play a role in your own aging and developmental path? Are you at the beckoning mercy of your genetic inheritance or the environment that surrounds you? Some theorists see humans as playing a much more active role in their own development. In this regard, humans possess agency, or the ability to make decisions and choices in order to fulfill aims and goals throughout life. Those favoring an active form of development view the human as agents of change. Classic developmentalists, such as Piaget, maintained the philosophy that human beings actively explore their world at a young age and construct new ways of thinking to explain the things they experience later in life. In contrast, many behaviorists such as Erikson maintained view of that that human interactions within the social environment were somewhat more essential to the developmental process. To this regard, humans are believed to be passive recipients of the environmental contexts in which they interact. Thus, the environment motivates and shapes human actions rather above and beyond the human agent alone.
Disease vs. Process
Is human aging a disease or a process? Some theorists view aging as a disease, or something that can ultimately be diagnosed, treated, and cured. Modern-day theorists, such as Aubrey DeGrey and David Sinclair believe that aging is ultimately reversible. In other words, some experts believe common chronic and lethal disease arising with age, such as heart disease, cancer, and dementia just to name a few can be stopped with the proper detection and intervention. Yet, other experts such as Jay Olshansky and Bruce Carnes have noted that aging is a process and cannot be stopped without the complete absence of disease. Therefore, human must learn to adjust and adapt by engaging in preventative measures that help delay or slow outcomes of aging in order to increase the number of quality days one lives.
Disengagement vs. Activity
Do humans desire more time to be alone in old age to process and find meaning the happenings of life, or would they rather remain socially engaged in the present. Cumming and Henry (1961) originally proposed that human beings prefer to disengage from their traditional roles in society as they reach advanced older age. Such behavior is believed beneficial to the individual who desires more time to appraise the meaning of life; as well as to the younger or next generation of society who seeks to new opportunity and advancement in roles once held by those who were older. However, Neugarten asserted (1964) that contentment in old age largely depends on active maintenance of forging on-going and new personal relationships, as well as engaging in the fulfillment of new social roles and endeavors which one enjoys or did not previously have the opportunity in which to engage during the early and middle years of life.
Mind vs. Body
Is aging and human development a matter of mind over matter? There is growing interest evolving from the world of neuroscience to better understand the interconnection between the aging mind versus the aging body. While some experts follow a more reductionistic approach via identification of specific genes, nerve pathways, imaging and or structural brand damage connected to decrements in one’s ability to walk, maintain balance, or react to environmental stimuli; others seek a more holistic explanation relative to pinpointing the interconnection between brain activity and performance of everyday activities of daily living, brain fitness activities and risk of dementia, and mindfulness practices (meditation, spirituality) and body awareness in time and space.
Causation vs. Association
How much of our development as humans is due to age versus something else? Of primary concern is understanding to what extent developmental outcomes are due to largely to age or being old. In other words, is age the cause and determinant of one’s physical and functional health, cognitive status and memory, or social preferences and relationships. Of additional interest is understanding to what extent developmental outcomes might be due variables beyond human aging. For instance, some experts commonly inquire as to what extent human development is less about growing old and more about other variables such as family heredity of genetics, biological sex, race/ethnicity, gender identity, education, geographic context, and other ecological factors that might come into play.
Three Theoretical Paradigms of Aging and Human Development
There are three prominent paradigms, or “worldviews of how phenomenon operate,” that guide how aging and developmental science experts develop theory for scientific inquiry (see Table 2 summary).
| Paradigm | Source | Causation | Process | Analysis | Premise |
|---|---|---|---|---|---|
| Organismic | Organism | Cells | Universal | Reductionistic | Programmed |
| Genetics | Random | ||||
| Environment | |||||
| Mechanistic | Social | Environment | Discontinuous | Additive | Social |
| Genetics | Cognitive | ||||
| Psychological | |||||
| Contextual | Ecological | Genetics | Continuous | Interactive | Life-History |
| Environment | Life-Span | ||||
| Socio-historical | Life-Course |
It is not unusual to find gerontological or geriatric experts relying on one or a combination of three perspectives to understand and explain age-related phenomenon that contribute to success in biological, psychological, and social development. Those who promote an organismic paradigm are often interested in studying the biology of the human organism relative to cellular and molecular functioning, genetics, and environmental exposures. A key assumption of this paradigmatic thinking is that human aging and development is a universal process that can be reduced to one analytical explanation linked to programmatic or random process as the human organism ages within their respective environment. Aging and developmental theorists following a mechanistic paradigm tend to focus less on the organism and emphasize the social environment as having a significant impact on aging and human development outcomes. Such experts are often interested in understanding how human interactions within the social environment contribute additive differences and similarities in maturational abilities and psychological outcomes across various age and stages of life. Of additional interest is understanding discontinuous processes or age-development stage human experiences and behaviors within social environments is interconnected with genetic expression as observed through psychological (e.g., identity/personality, positive/negative emotion, intellect and learning, or wisdom), as well as social (e.g., romance and marriage, friendship, generativity) outcomes. A final foundational paradigm concerns the broader context. Aging and developmental theorists who endorse the contextual paradigm consider humans as living organisms embedded within the ecology of the social environment, which serve as a dynamic and interactive gateway by which genes are expressed, environments influence behavior, and individuals and populations uniquely respond and cope to socio-historical experiences within the cultural context or system. Based on the contextual paradigm, it is assumed that aging and development is continuously altered for better or worse by human biology, psychological risk and resilience, and socio-historical timing.
Biological Theories of Aging
Biological theories of aging fall under two categories of thinking:
- Programmed
- Random Error
Programmed theories of aging follow a biological timetable, possibly a continuation of infant and childhood development. This timetable is believed to be linked to changes in gene expression that affect systems responsible for the maintenance, repair, and protection of the body. Thus, programmed theories of aging posits that human aging, particularly at the cellular level contribute to biological changes which are genetically pre-determined or set across the course of time or the life-span of the organism. On the other hand, random error theories of aging focus on chance exposure to environmental stressors which causes accidental risk or random cellular damage at the molecular level, which ultimately lead to catastrophic age-associated disease or even genetic mutations.
Have you ever wondered to what extent biology or genetics explains how long different species live? No species is capable of living forever. Yet, a key rationale in support of programmed theories of aging comes from the fact that the average life-spans across various species is highly variable. For instance, the common house mouse is capable of living up to 4 years, domesticated dogs can potentially live to be roughly 30 years, domesticated cats can survive 38 years, and horses can live a maximum of 57 years; however giant tortoises have a life span of approximately 180 years. In fact, the world’s oldest tortoise named Jonathan recently celebrated his 190th birthday.
Video: Jonathan, St. Helena’s ancient tortoise, awaits visitors (2:00)
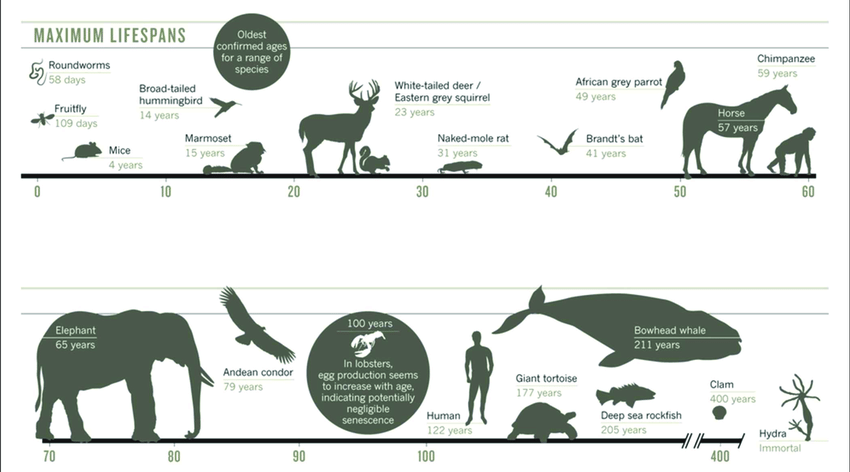
Meanwhile, humans have the potential to live 122 years; a record set by Jeanne Louise Calment of Arles, France, who lived to be 122 years and 164 days.
Programmed Theories of Aging
Gompertz’s Equation
One of the earliest programmed theoretical approaches applied to aging was the Gompertz Equation, a statistical function used to plot and compare the association between age and death rates of a given species. The creator of this statistical process was Benjamin Gompertz, an 18th century British mathematician and actuary who applied calculus to mortality data to demonstrate that death increases in geometric progression at increasing ages (Gompertz, 1825). In other words, the longer a human being lives, there chances of survival decrease while their chances of death increase. The Gompertz Equation is still considered a relevant calculation today and is widely use as a statistical comparison of human populations within the field of biodemography, a sub-discipline of gerontology-geriatrics combining demography, epidemiology, and biology to understand population-based patterns related to fertility, survivorship, longevity, and death. Of central focus is understanding population aging, or the median age in a given population based on fertility and life-expectancy rates, as well as population longevity, or the estimate of survival versus death within a given population of persons.
A second credible and classical theory of programmed aging included Raymond Pearls’ (1928) rate of living theory, in which basal metabolic rate or metabolism was theorized to be causally associated with an organism’s life-span. One key assumption is that as metabolic rate increases; the lifespan of an organism was expected to decrease. However, a 2007 study using modern statistical methods to correct and control for the effects of body size and phylogenetic contrasts indicated that metabolic rate does not necessarily correlate or translate into a longer life for mammals, including humans (de Magalhães, Costa, & Church, 2007). Yet, the jury is still not out relative to the theoretical role of metabolism in human aging, which by some accounts remains essential to how human aging is programmed and controlled biologically.
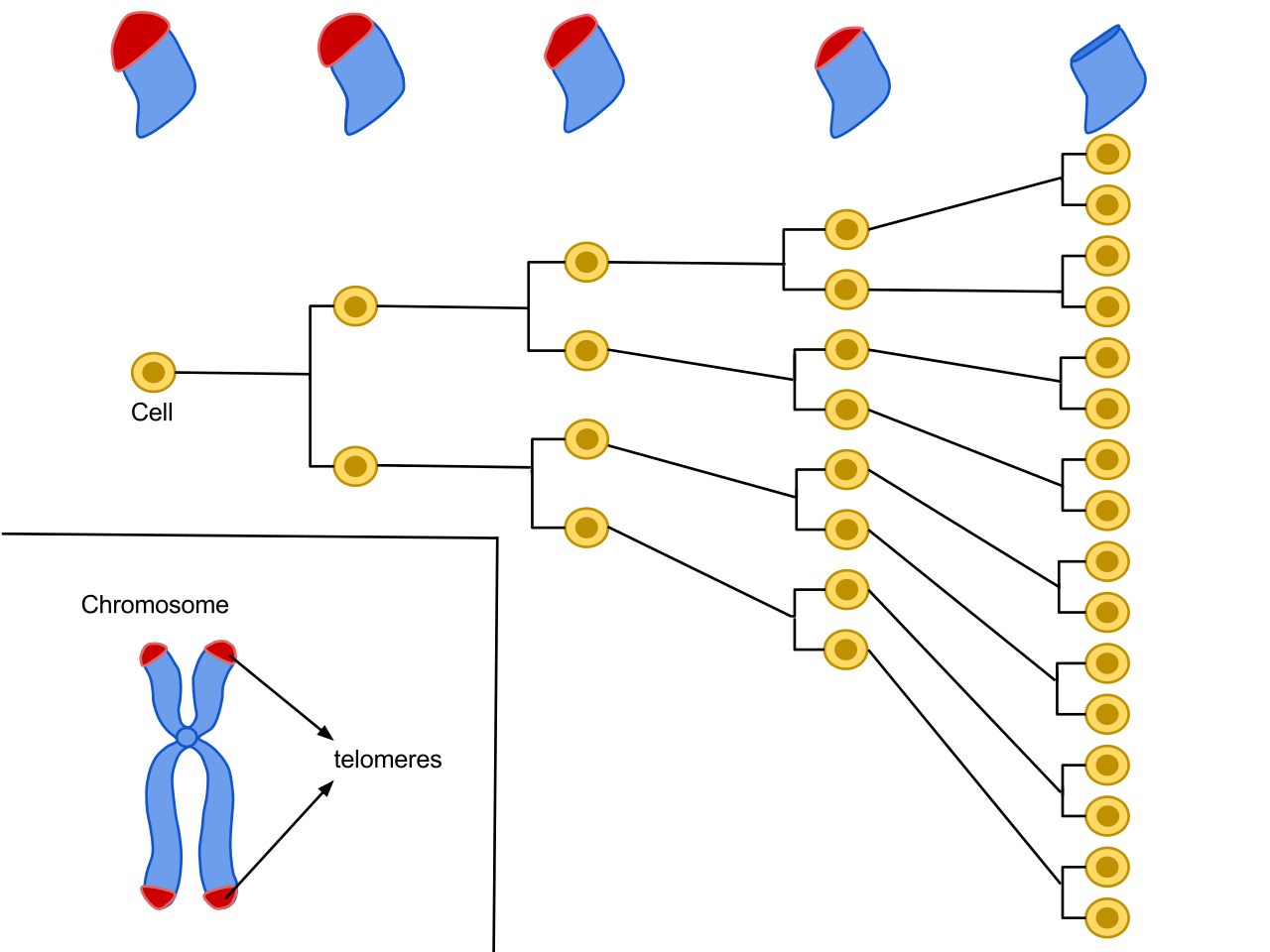
Replicative Senescence (Hayflick Limit)
Modern-day programming theories of aging have focused on senescence, or the cellular processes of growing old. Cellular senescence is a process of by which cells age, stop dividing, an die. It is believed that large numbers of old or senescent cells build-up in tissues and organs of the body, which ultimately contributes to disablement and diseases leading to the death of the organism. Replicate senescence refers to the process by which normal somatic cells reach an irreversible stage of death following multiple rounds of division and replication. The final stage of cell live is associated with notable changes to genetic expression and organism functioning. Leonard Hayflick advanced theoretical understanding of cellular senescence by discovering that normal cultured human cells have a pre-set and limited capacity to replicate and divide 40-60 times after which they become senescent; a phenomenon known as the Hayflick Limit. Hayflick’s discovery further propelled theoretically understanding of the effects and connection of cellular aging to genetics.
Telomere Theory
The telomere theory of aging is one such theory built upon the concept of replicative senescence or the Hayflick Limit (see Figure 2.2). A telomere is believed to be a protective sheath or coating on the ends of chromosomes. Chromosome contain DNA or genetic information which vital to human health and protection against illness and disease. Telomere theory proposes that exposure to environmental stressors, as well as appraisal of life-event stressors due to normative aging processes erode and shorten telomere length over time (Méndez-Chacón, 2022; Rehkopf et al., 2013; Ruiz-Narváez et al., 2021; Sanders & Newman, 2013). This biological timing of telomere shortening is further hypothesized to be pre-set. As humans age across mid-life and into old and very old age, the telomere progressively shrinks until the ends of chromosomes are no longer protected and our DNA or genes are exposed. This leads to cellular senescence and critical transformation of somatic cells which results in irreparable biological damage to the organism. The end-result includes diminished health functioning, increased incidence of chronic diseases such as heart disease, cancer, dementias, and other pathological processes of aging, and reduced life-span or chances of further survival.
FOX Gene Theory
Among humans, researchers have estimated roughly 25% of longevity is genetic or inherited. However, this estimate genetic influence increases among life-spans over 60 years of age. Sabastiani & Perls (2012) estimated that genetics may explain as much as 33% of life-span among women and 48% among men who survive to age 100. This brings to question whether there is an aging or longevity gene responsible for biological robustness. The FOXO gene theory proposes that there are a group of genes called the FOXO genes, which place a critical role in regulating cellular processes regulating stress, metabolism, and the cycle of cellular life. FOXO is referred to the the forkhead box (FOX) family of genetic transcription factors, with forkhead referring to its’ winged or butterfly-like helix structure (Hannenhalli & Kaestner, 2009). In particular, the FOXO3 gene variant had been dubbed as the “longevity gene” (Wilcox et al. 2008). A convincing amount of empirical evidence suggests that FOXO3 is a key genetic regulator of age-associated diseases such as hypertension, cardiovascular disease, cancer, and dementias (Donlon et al., 2022; Nakagawa et al., 2022; Morris et al. 2015;). There is also increasing empirical support that FOXO3 may differentially pre-determine the biological onset, timing, and progression of such age-related diseases by gender and across various racial and ethnic groups (Wilcox et al., 2017). Recent report further suggests that FOXO3 serves as the genetic control of telomere attrition during the aging process, a hypothesis that has gained some recent empirical interest and will likely continue to be investigated (Allsopp et al., 2019).
Random Error Theories of Aging
Ever hear someone express or imply that feel as if they are “falling apart” or “wearing out” they older they get? One of the earliest classical random error theories was devised by German biologist, August Wiesmann who proposed that aging results from a gradual deterioration of the cells and tissues via “wear and tear.” Known as the wear and teary theory of aging, Wiesmann theorized that aging is symptomatic of progressive damage to the cells and bodily systems over time due to an array of internal and external factors such as exposure to environmental toxins, radiation other sources which can harm cells and eventually damage genes. Over time the human body “wears out” due to overexposure as well as overuse resulting in disease and disablement that renders humans unable to biologically function correctly. According to this perspective, our bodies are somewhat like machines, such as a car. With repeated use, regular maintenance is required from a trained repair specialist for proper short and long-term functioning. Normative structural issues and occasional accidental damage may periodically arise and require repair and replacement of damaged or non-functional parts. With some general diagnostics, installation of new parts, and repair, things should operate like new or normal. However, one must also pay attention to everyday exposure within the environment and due to climate, which can further create stressors resulting in gradual decrements, accidental damage, and even errors in proper functioning. In contrast, programmed errors theories would argue that aging is not linked to random or chance encounters that may contribute to accident, damage, or error; rather the human body was not built to last and therefore must systematically deteriorate in an expected pre-determined manner.
Cross-Linking Theory
You likely know someone who has complained of stiffness of the back, knees, toes or fingers. Perhaps, you may have also heard them complain about bending over, standing up after having sat in a chair, or having to walk a short distance. One of the commonly known Random Error Theories in the Biology of Aging includes cross-linking theory. Cross-linking theory proposes that normative aging causes detrimental changes to cells that make up connective tissues within the human body, such as cartilage, tendons, skin, muscles, and bones. Cross-linking develops within collagen, a fibrous protein in the body. Collagen is responsible for provide flexibility, elasticity, and strength to bodily tissues for purpose of contributing to mechanical functions such as sitting, standing, stretching or walking. The molecular make-up of collagen consists of three strands or chain of amino acids wound together into a tight helix structure. Similar to the structure of a ladder, the strands of collagen are horizontally attached to the strands of protein. With increasing age, each strand starts to become interconnected with the other causing the molecules to shrink size and become increasingly rigid. In the midst of this process, consumption and in-take of various sugars leads to a process known as glycation. This believed to contribute the formation what is labeled as Advanced Glycation End-Products or AGEs over time (Boaz & Moshe, 1998). AGEs initiate progression of cross-linking collagen which results in increase stiffness of muscles, joints, tendons, and cartilage. It is believed that diet and lifestyle habits play an important role relative to the onset, progression, and impact of AGEs on human biological functioning and diseases linked to inflammation, arthritis, and diabetes (Chuah et al., 2013; de Groot, 2011; Ott et al., 2014). Recent evidence testing cross-linking theory appears to suggest that long-term consumption of dietary sugars represents a mechanism by which AGEs contribute to cumulative biological damages in the form of disease (Aragno & Mastrocola, 2017).
Free Radical/Oxidative Stress Theory
Another theory focuses on free radicals, or unstable oxygen molecules that are produced when cells create energy. A free radical thrives by seeking out and binding to other molecules. Mitrocondria, a cell organelle that uses oxygen to produce energy for food, is vital to converting oxygen to adenosine triposhphate (ATP) and providing the cell with energy. When the mitochondria uses oxygen to produce energy, they also produce potentially harmful by-products called oxygen free radicals (NIA, 2011a). The free radicals are missing an electron and create instability in surrounding molecules by taking electrons from them. There is a snowball effect (A takes from B and then B takes from C, etc.) that creates more free radicals which disrupt the cell and causes it to behave abnormally (See Figure 2.3).
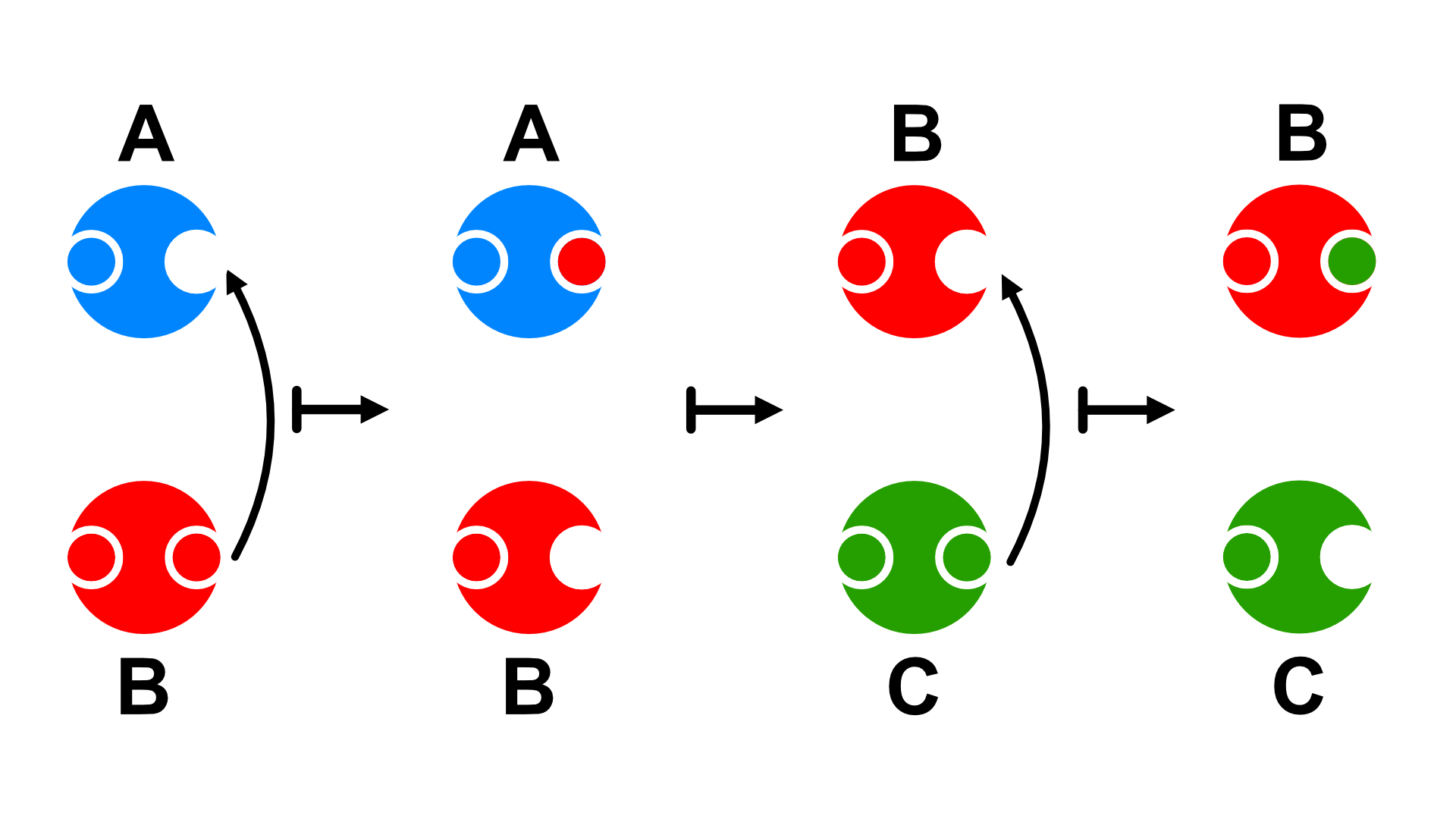
Some free radicals are helpful as they can destroy bacteria and other harmful organisms, but for the most part they cause damage in our cells and tissue. Free radicals are identified with disorders seen in those of advanced age, including cancer, atherosclerosis, cataracts, and neurodegeneration. Some research has supported adding antioxidants to our diets, including consumption of various vegetables, fruits, and nuts, to counter the effects of free radical damage because the antioxidants can donate an electron that can neutralize damaged molecules. However, the research on the effectiveness of antioxidants is not conclusive (Harvard School of Public Health, 2016).
Alternatives to free radical theory itself have focused on explaining the role of antioxidants as contributors to the rate of aging. One plausible hypothesis involves the concept of caloric restriction, the view that limiting consumption of daily dietary calories can improve health and prolong life with or without exercise (Coleman, 2014; Walford et al., 2002). Another plausible explanation of free radical theory suggests that resveratrol, a natural compound found in grapes and particularly red wine, is a highly potent antioxidant (Pearson et al., 2008). However, the amount of wine human would need to consume on a daily basis far exceeds that which would be necessary to have immediate benefits to protection against age-associated disease. Scientists remain interested in the effects of resveratrol and believe the age benefits may be comparable to that of caloric restriction.
Hormonal Stress Theory
Hormonal Stress Theory, also known as Neuroendocrine Theory of Aging, suggests that as we age the ability of the hypothalamus to regulate hormones in the body begins to decline leading to metabolic problems (American Federation of Aging Research (AFAR) 2011). This decline is linked to excess of the stress hormone cortisol. While many of the body’s hormones decrease with age, cortisol does not (NIH, 2014a). The more stress we experience, the more cortisol released, and the more hypothalamic damage that occurs. Changes in hormones have been linked to several metabolic and hormone related problems that increase with age, such as diabetes (AFAR, 2011), thyroid problems (NIH, 2013), osteoporosis, and orthostatic hypotension (NIH, 2014a).
Autoimmune Theory
The autoimmune theory of aging proposes that aging is due to faulty immune system functioning. In particular, the immune system attacks the body’s own cells. Lifetime exposure to stress affects how e age. Aging. It is believed that human possess an innate and adaptive immune system vital to healthy aging. The innate immune system is made up of the skin, mucous membranes, cough reflex, stomach acid, and specialized cells that alert the body of an impending threat. With age these cells lose their ability to communicate as effectively, making it harder for the body to mobilize its immune defenses. Meanwhile, the adaptive immune system includes the tonsils, spleen, bone marrow, thymus, circulatory system and the lymphatic system that work to produce and transport T cells. T-cells, or lymphocytes, fight bacteria, viruses, and other foreign threats to the body. T-cells are in a “naïve” state before they are programmed to fight an invader and become “memory cells,” or cells that remember how to fight a certain infection should the body ever come across this invader again. Memory cells can remain in your body for many decades, and why the measles vaccine you received as a child is still protecting you from this virus today. As older adults produce fewer new T-cells to be programmed, they are less able to fight off new threats and new vaccines work less effectively. The reason why the shingles vaccine works well with older adults is because they already have some existing memory cells against the varicella virus. The shingles vaccine is acting as a booster (NIA, 2011a). Although the autoimmune theory of aging does not provide a broad enough explanation to address the rate or process of aging, it does deserve mention as a plausible explanation as to why certain prevalent diseases in old age such as, arthritis or lupus, are inadvertently diagnosed despite limited family health history for certain diseases. One example is Sjögren’s Syndrome, a chronic autoimmune disorder that commonly attacks the glands that produce tears, saliva, and joint and muscle pain. This particular auto-immune health issue commonly impacts women more than men of all ethnic and racial background starting around 50 to 50 years of age (https://www.niams.nih.gov/health-topics/sjogrens-syndrome) .
Error Catastrophe
In animal models, mice with mutations in the mitochrondrial have been reported to show accelerated signs of aging, including graying and hair loss, muscle wasting, and reduced bone and spinal mass and strength (Kukat & Trifunovic, 2009; Ma et al., 2018). Such findings seem to be translated to the experience of human aging. There is further evidence that exposure to environmental stress may alter a process known as DNA methylation, or amino acid sequencing within a gene. This is believed to have unique effects during fetal development, include maternal stress experiences during pregnancy which can alter genetic expression across the biological life-span of the child; thus increasing susceptibility to disease more prevalent in later adulthood. In effect, our exposure to the environment entails an interaction with genetic timing and predisposition to influence how individuals age at varying rates. Progeria, also known as Hutchison-Gilford syndrome is a very rare type of genetic mutation that occurs in fetal development and impact. Progeria is a mutated and fatal disease that causes individuals, particularly children, to rapidly age faster than usual. Most of the time, these young children appear older than they are as evidenced by early age-associated symptoms such as wrinkled skin, graying and loss of hair, vision and hearing loss, joint stiffness and pain, and musculoskeletal dengeneration. Children with progeria usually have a normal appearance in early infancy with the signs and symptoms of accelerated aging appearing around 9-24 months. Humans born with progeria live approximately 14-15 year with heart disease being the primary cause of death. However, some children diagnosed with Progeria can live 20 or more years.
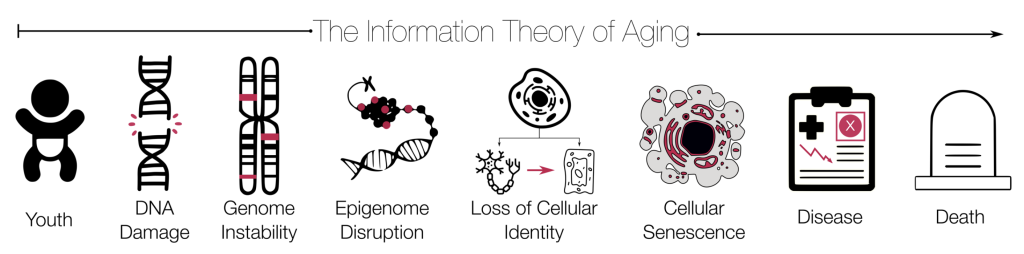
Information Theory of Aging
There is growing evidence in support of the Information Theory of Aging (Karnaukhov et al., 2017; Sinclair, 2019; 2023; Vujin & Dick, 2020). This theory suggests that biological aging results from an accumulation of errors in an organism’s genetic code over the course of its’ lifespan, which leads to an genetic instability and an epigenome disruption or loss of cellular expression and identity. In other words, this disruption results in an inability of genes to “turn-on” or “turn-off.” In effect, this theory assumes that the loss of epigenetic or non-DNA encoded gene regulatory information is the principal cause of human aging (Vujin & Dick, 2020). one principal central biological cause of aging. As cells divide and replicate, genetic information is subject to errors, including mutations and epigenetic changes. The accumulation of genomic damage contributes to decreased cellular repair functioning. In other words, genetic damage renders cells ineffective and increases probability of organism death. The Information Theory of Aging affirms that human aging is a form of “error catastrophe,” most notably associated with a critical tipping point during which accumulated errors in genetic code activate decline in biological functioning. The information theory of aging further posits the that molecular modifications occurring on the surface of DNA, acts as secondary layer of genetic control vital to cellular growth and development. According to Sinclair (2019), if assumptions of Information Theory of Aging hold true, it is possible that individuals will one day have greater control over age-associated diseases, as well as enjoy the prospect of cheating death to a certain extent.
David Sinclair: An Information Theory on Aging (Audio, 48:26)
Life History Theory
Life history theory is a contemporary theory that combines underlying and associated concepts surrounding programmed versus random-error biological perspectives in aging with evolutionary biology, a subdiscipline of biological science focused on the origins, diversification, and adaption of life over time. Life history theory is often used as a framework for understanding the genetic and environmental interactions (i.e. Gene X Environment) that contribute to increased as well as decreased survivorship and longevity across various populations of people. It has also be used as a relevant explanatory basis of the mechanisms by which certain genetics, behavioral expressions, and environmental conditions may protect certain persons or populations against common age-associated diseases.
Central to life-history theory is the concept of natural selection, or differential survival and reproduction of individuals due to heritable or selective traits that become more common within population resulting in biological advantages connected to human development, aging, and survival. This is often dependent on fitness, or reproductive success or propensity to produce offspring across the lifetime of the individual. Of particular interest is theoretically understanding how organisms living under various environmental conditions are differentially challenged by fundamental tradeoffs in their ability to pass along two key heritable traits vital to aging and biological development: (1) genotypes or an organism’s genetic code by way of DNA and; (2) phenotypes, observable physical, structural, and behavioral traits or characteristics necessary for continued environmental adaptation and survival. One primary assumption of life-history theory contends that any mismatch between genetic expression and traits that may have previously benefited biological aging in one environment may no longer be adaptive or beneficial to the offspring’s biological aging process or chances of survival in a current or different environment. Thus, it is believed that the environment play an influential role in determining the extent which offspring may or may inherit certain genes, the timing by which genetic traits may be “turned-on” or “turned-off” across the lifetime of the organisms, as well as the extent which various phenotypes may or may not be expressed.
Del Giudice and colleagues (2015) highlighted three trade-offs or biological assumptions surrounding the developmental trajectory of human aging and survival. These assumptions include the (1) present-future reproduction trade-off or the differential costs and benefits to fertility or mortality associated with deciding to have offspring at a younger age at the expense of delaying the timing of reproduction and having offspring at in the future or at an older age; (2) quality vs. quantity of offspring tradeoff or the idea that high individual investment in reproducing and raising offspring usually results in the birth of offspring with higher quality biological or genetic traits; whereas limited individual investment or concern regarding reproduction and raising offspring results in a greater number of offspring with lower quality biological or genetic traits and; (3) the mating effort versus parenting tradeoff whereby greater parental effort and decision to invest resources (i.e., time, energy, money) toward offspring already born contributes to a slower life history strategy (i.e., decision to invest and distribute resources to fewer children); however or greater effort and investment of resources to conceive more offspring coincides with a faster life history strategy (i.e., decision to invest, divide, and distribute resources across greater number of children).
Maternal-offspring advantage versus disadvantage can help illustrate the trade-off assumptions pertaining to life-history theory. For instance, adult woman who is actively engaged in reproduction and having multiple children across younger ages may actually reduce their own biological potentials for growth, maintenance, and chances of survival across later adulthood; yet the offspring will experience a gains potential biological growth and expected number of years to live (Gavrilov et al., 1997; Gavrilov & Gavrilova, 2001; 2011; 2015). Yet, an adult woman who decides to safeguard their own biological potentials by delaying childbirth, subsequently confines fertility to a fewer numbers of years at an older age, when they are ready to invest in reproduction and children. Maternal delay of childbirth is associated with an increased probability that the mother will live longer, but the offspring is less likely to experience the biological benefit of increased longevity (Gavrilov et al., 1997; Gavrilov & Gavrilova, 2001; 2011; 2015). What is might be a plausible explanation for the biological and development outcomes associated with such trade-offs? For starters, adult women who engage in tasks of fecundity or frequent childbirth at an earlier ages, may be simultaneously compromising their own survival relative to the cost to their immune functioning, particularly in instances where there are environmental risks in the season or timing of conception (e.g., exposure risk to season diseases such as the common cold or influenza; Gavrilov et al., 1997; Gavrilov & Gavrilova, 2011). Although the fetus receives genetic benefits of enhanced immune protection from the mother; the mother’s immunity may actually be reduced. In addition, some adults may not survive long enough to feel they have reached full sexual maturity and personal responsibility to reproduce or have a child (Garvrilov & Gavrilova, 2015). In such cases, an opportunity for heritability of genotypes or expression of varying phenotypes is lost. Finally, if the mother is spending a substantial amount of time nurturing, feeding, and caring for a fewer number of existing offspring, this will potentially increase the quality of genetic heritability) and phenotype expression to existing offspring. Yet, it might be that such investment may be physiologically demanding and stressful resulting in diminished biological functioning and reduced survival, as well as any future chances to extend one’s fertility and reproduction.
Key Takeaways
Important concepts from this chapter include, but are not limited to, the following:
- Gerontology and Geriatrics are related, but distinct and separate disciplines
- The aging process is not fully understood, but several theories help explain what it is and why it happens. This includes dualities like Nature vs. Nurture, Continuity vs. Discontinuity, Active vs. Passive, Disease vs. Process, Disengagement vs. Activity, Mind vs. Body, and Causation vs. Association.
- There are three prominent paradigms, or worldviews of how phenomenon operate, that guide how gerontologists develop theory for scientific inquiry: Organismic, Mechanistic, and Contextual.
- Biological theories of aging fall under two categories: Programmed and Random Error
- Benjamin Gompertz’s equation states that chances of survival decrease while chances of death increase
- Genetic theories of aging include Replicative Senescence, Telomere Theory, and FOX Gene Theory
- Random Error theories of aging are based in the idea that the human body wears out over time
- Life History Theory is often used as a framework for understanding the genetic and environmental interactions that contribute to aging
References
Allsopp, R. et al. (2019). The longevity associated allele of FOXO3 protects against telomere attrition during aging. Innovations in Aging, 3 (Suppl 1), S99-S100. https://doi.org/10.1093%2Fgeroni%2Figz038.374
Arangno, M., & Mastrocola, R. (2017). Dietary sugars and endogenous formation of advanced glycation endproducts: Emerging mechanisms of disease. Nutrients, 9(4), 385. https://doi.org/10.3390/nu9040385
Boaz, L., & Moshe, W. J. (1998). Long-term fructose consumption accelerates glycation and several age-related variables in male rats. The Journal of Nutrition, 128 (9), 1442-1449. https://doi.org/10.1093/jn/128.9.1442
Chuah, Y. K., Basir, R., Talib, H., Tie, T. H., & Nordin, N. (2013). Receptor for advanced glycation end products and its involvement in inflammatory diseases. International Journal of Inflammation, Vol. 2013, Article ID 403460. https://doi.org/10.1155/2013/403460
Clancy, S. et al. (2008). Translation: DNA to mRNA to protein. Nature Education, 1, (1). https://www.nature.com/scitable/topicpage/translation-dna-to-mrna-to-protein-393/
Coleman et al. (2014). Caloric restriction reduces age-related and all-cause mortality in rhesus monkeys. Nature Communication, 5, 3557. doi:10.1038/ncomms45557.
Cumming, E., & Henry, W. E. (1961). Growing old, the process of disengagement. Basic books.
de Groot, L., Hinkema, H., Westra. J., Smit, A. J., Kallenberg, C. G. M., Bijl, M., & Posthumus, M. D. (2011). Advanced glycation endproducts are increased in reheumatoid arthritis patients with controlled disease. Arthritis Research & Therapy, 13. Article R205(2011). https://doi.org/10.1186/ar3538
Del Giudice, M. et al. (2015). Life history theory and evolutionary psychology. In D. M. Buss (Ed.), The handbook of evolutionary psychology (foundations 2nd ed., Vol. 1, pp 88-114). Hoboken: Wiley.
Donolon et al. (2022). FOXO3, A resilience gener: Impact on lifespan, healthspan, and deathspan. The Journals of Gerontology, Series A, 77 (8), 1479-1484. https://doi.org/10.1093/gerona/glac132
Garvilov, L. A. et al. (1997). Maternal age and lifespan of offspring. Doklady Akademi Biological Sciences, 354 (4), 569-572.
Garvilov, L. A., & Garilova, N. (2001). Biodemographic study of familial determinants of human longevity. Population: An English selection, 13, 197-221.
Gavrilov, L. A. & Gavrilova, N. (2011). Season of birth and exceptional longevity: Comparative study of American centenarians, their siblings, and spouses. Journal of Aging Research. Vol. 2011. Article ID 104616. Doi: 10.4061/2011/104616.
Garvrilov, L. A., & Gavrilova, N. S. (2015). New developments in the biodemography of aging and longevity. Gerontology, 61(4), 364-371.
Goldhaber, D. E. (1999). Theories of human development: Integrative perspectives (1st edition). New York, NY: McGraw-Hill.
Gompertz, B. (1825). XXIV. On the nature of the function expressive of the law of human mortality, and on a new mode of determining the value of life contingencies. In a letter to Francis Baily, Esq. FRS &c. Philosophical transactions of the Royal Society of London, (115), 513-583.
Hannehalli, S., & Kaestner, K. H. (2008). The evolution of Fox genes and their role in development and disease. Nature Reviews Genetics, 10, 233-240. https://doi.org/10.1038/nrg2523
Karnaukhov, A. V. (2017). The information theory of aging: The major factors that determine lifespan.Biophysics, 62 (5), 829-835.
Kukat, A., & Trifunovic, A. (2009). Somatic mtDNA mutations and aging- facts and fancies. Experimental Gerontology, 44(102), 101-105. https://doi.org/10.1016/j.exger.2008.05.006
Li, J. et al. (2017). A comparative study of anti-aging properties and mechanism: Resveratrol and caloric restriction. Oncotarget, 8(39), 65717-65729. https://doi.org/10.18632/oncotarget.20084
Ma, H. et al. (2018). Germline and somantic mtDNA mutations in mouse aging. PloS ONE, 13(7), e0201304. doi:10.1371/journal.pone.0201304
de Magalhães, J. P., Costa, J. & Church, G. M. (2007). An analysis of the relationship between metabolism, developmental schedules, and longevity using phylogenetic independent contrasts. Journals of Gerontology A: Biological and Medical Sciences, 62(2), 149-160. https://doi.org/10.1093/gerona/62.2.149
Méndez-Chacón, E. (2022). Gender differences in perceived stress and its relationship to telomere length in Costa Rican adults. Frontiers in Psychology, 13. https://doi.org/10.3389/fpsyg.2022.712660
McCune, S., & Promislow, D. (2021). Healthy, active aging for people and dogs. Frontiers in Veterinary Science, 570.
Morris, B. J., Wilcox, D. C., Donion, T. A., & Wilcox, B. J. (2015). FOXO3 – A major gene for human longevity. Geronotology, 61(6), 515-525. doi: 10.1159/000375235
Nakagawa, K., et al. (2022). Forkhead box O3 longevity genotype may attenuate the impact of hypertension on risk of intracerebral haemorrhage, Journal of Hypertension, 40 (11), 2230-2235. doi:10.1097/HJH.0000000000003249
Neugarten, B. L. (1964). Personality in middle and late life: Empirical studies.
Ott, C., Jacobs, K., Haucke, E., Santos, A. N., Grune, T., & Simm, A. (2014). Role of advanced glycation end products in cell signaling. Redox Biology, 2, 411-4129. https://doi.org/10.1016/j.redox.2013.12.016
Pearl, R. (1928). Experiments on longevity. The Quarterly Review of Biology, 3(3), 391-407.
Rehkopf, D. H., Dow, W. H., Rosero-Bixby, L., Lin, J., Epel, E. S., & Blackburn, E. H. (2013). Longer leukocyte telomere length in Costa Rica’s Nicoyan Penisula: A population-based study. Experimental Gerontology, 48(11). https://doi.org/10.1016/j.exger.2013.08.005
Ruiz-Narváez, E., Baylin, A., Azofeifa, J., Leal, A., & Rosero-Bixby, L. (2021). Diet and leukocyte telomere length in a population with extended longevity: The Costa Rican Longevity and Healthy Aging Study (CRELES). Nutrients, 13(8). doi 10.3390/nu13082585
Sander, J. L., & Newman, A. B. (2013). Telomere length in epidemiology: A biomarker of aging, age-related disease, both, or neither? Epidemiologic Reviews, 35, 1, 112-131. https://doi.org/10.1093/epirev/mxs008
Sebastiani, P., Solovieff, N., DeWan, A. T., Walsh, K. M., Puca, A., Hartley, S. W., & Perls, T. T. (2012). Genetic signatures of exceptional longevity in humans. PloS one, 7(1), e29848.
Sinclair, D. A, & LaPlante, M. (2018). Lifespan: Why we age-and why we don’t have to. New York, NY: Atria Books.
Speiser, J. L., Callahan, K. E., Houston, D. K., Fanning, J., Gill, T. M., Guralnik, J. M., & Miller, M. E. (2021). Machine learning in aging: an example of developing prediction models for serious fall injury in older adults. The Journals of Gerontology: Series A, 76(4), 647-654.
Vujin, A. & Dick, K. (2020). The information theory of aging: Hacking immortality? Health Science Inquiry, Vol. 11. https://doi/10.29173/hsi304.
Wilcox, B. J., Morris, B. J., Tranah, R. C., Masaki, K. H., He, Q., Wilcox, D. C. , Allsopp, R. C., Moisyadi, S., et al.(2017). Longevity-associated FOXO3 geneotype and its impact on coronary artery disease mortality in Japanese, Whites, and Blacks: A propspective study of three American populations. The Journals of Gerontology Series A, 72,(5), 724-728. https://doi.org/10.1093/gerona/glw196
Wilcox, B. J., Donlon, T. A., He, Q., & Curb, J. D. (2008). FOXO3A genotype is strongly associated with human longevity. PNAS, 105 (37). https://doi.org/10.1073/pnas.0801030105

