36 Computer Simulation: An Innovation in Teaching and Learning of Chemistry in Secondary Schools
Josephine C. Nnadi
Abstract
The study focused on computer simulation and as innovative and improvisation for the teaching and learning of chemistry in secondary schools in developing countries like Nigeria. It highlighted what simulation is; and how they enhance learning, foster high level of cognition and overcome misconceptions in chemistry. This paper also discussed how simulation motivate the learners, improve interest in and attitude to chemistry and support chemistry education generally. The challenges to and prospects of the use of simulation and animation in teaching and learning of chemistry in secondary schools in the twenty first century in developing countries were also discussed. Practical examples of computer simulation of chemical bonding were presented.
Introduction
Science and technology are important tools for development and productivity in any nation. In the world today, science and technology has become a dominant power development indicator (Agbaje & Alake, 2014). Science has been regarded as the base of modern day technological breakthrough. The study of science is of great importance in Nigeria, that a lot of emphasis has been laid on the teaching and learning of science. The major aim of science education as contained in the Federal Republic of Nigeria in her national policy on education is to equip the students to live effectively in this modern age (FRN, 2013). This can be achieved by the inculcation in the learners the necessary scientific skills and attitudes. The inculcation of scientific skills and attitudes in students can only be achieved through the proper teaching of the various science subjects like Chemistry.
Chemistry as one of the science subjects is taught at the senior secondary school level of Nigeria education system. Chemistry is the science of matter and the changes it undergoes, and attempts to explain chemical phenomenon of everyday life (Kolomuc & Tekin, 2011). Chemistry is essentially needed for a nation’s technological development. As a core science subject, the proper teaching and learning of chemistry in secondary school facilitate student’s enrollment in many professional discipline like Nursing, Medicine, Engineering, Agriculture and Geology among others.
Despite the importance of chemistry in science and technological development, students’ achievement in the subject at senior school certificate examination (SSCE) has been consistently poor in both internal and external Examination (Giginna, 2013).
West African Exam Council (WAEC) Chief Examiner reports May/June 2010, 2011, 2012, 2013 and 2014 for instance attest to this ugly performance of chemistry student’s in external exam. The report revealed that the pass rate at credit level in chemistry were 15%, 18%, 21%, 31.28% for 2010, 2011, 2012, 2013 and 2014 respectively.
Also according to West Africa Examination Council (WAEC, 2010), the use of conventional approach is deficient in meeting the needs of new instructional strategies in teaching and learning. In this computer age numerous Information Communication and Technology (ICT) applications are available. They stimulate students’ active engagement in teaching and learning process. Among the various ICT applications, computer simulation is of special importance in chemistry teaching and learning. The use of educational technology like simulation for instance in science classroom, not only helps with students’ understanding of content, but positively impacts student’s engagement in lesson and their attitude towards learning (Shu –Nu, Yau- Yuen & May 2009).
Simulation
Computer simulation has been defined in different ways by different researchers. According to Alessi and Trollip (2001), simulation is just one type among many of computer assisted instruction (CAI). Simulations are tools that facilitate learning through representation and practice in a repeatable, focused environment (Aldrich, 2004). According to Goldsim (2011), Computer simulation helps to identify and understand factors which control the system and or to predict the future behavior of the system. Simulation includes role plays, games, computer programs that encourage students to become active participants in classroom.
Classification of simulation
Simulation can be classified in many ways:
- Physical simulation: Here the physical object is presented on a screen and the students learn about it. For an example when electrons are displayed to observe the influence of temperature. Here students can manipulate the temperature to see its effect in the movement of electrons.
- Process simulation: Processes that are not visible can be demonstrated using processing simulations. For example how population grows and declines or rise and fall of stock exchange.
- Procedural simulation: Here procedures are followed in order to understand sequence of event. Students can be asked to follow a set of procedures in observing reaction rate which ends in a particular product.
- Situational simulations: This has to do with attitudinal and behavioral changes of people. The students use this simulation to explore the effects of different approaches to a problem (Tippler, 2003).
Types of simulation
Simulation has three types namely,
- Live simulation: This shows human behavior in real life. Example is training of soldiers in war games.
- Virtual simulation: Simulation occurs in a computer controlled setting. For example a pilot flying an aircraft but is controlled from the control room.
- Constructive simulation: This does not involve human or equipment but by proper sequencing of events. For example weather changes like wind directions or water wave and be controlled through application of temperatures and pressures. (Institute of simulation and Training, (IST) University of Florida (2002)).
Merits of Computer Simulation
The following are the merits of computer simulation in teaching and learning process.
Enhancement and fast learning
Students have various learning styles. We have visual learners, auditory learners and kinesthetic learners. Gardner (1993), also observed that students have multiple intelligences and not all students learn best through the traditional teacher centered verbal instruction. The theory of multiple intelligences requires teachers to expand their repertoire of teaching tools and strategies, breaking free from the traditional linguistic and logical approaches. In support of this Suleiman (2011), posits that the use of interactive simulation can serve not only as a specific remedy to one sidedness in teaching, but also as an organizational tool that facilitates and complements existing educational pedagogy.
The use of interactive simulations is an approach to teach children at risk for learning difficulties because it requires active participation, is multi-sensory in nature, and has the ability to peak the interest of all children. Studies have shown that instruction in the science classroom should incorporate students being actively engage in the material in order for maximum achievement to occur. Students need to be able to take concepts from the science classroom and apply them to their everyday lives. Effective instruction needs to be relevant to students’ lives, not just facts that must memorize for test. Through the use of animations and simulations this connection can be bridged more effectively through traditional instruction. The effectiveness of educational technology and the belief that simulation help foster students’ engagement, perceptions and learning is much realistic in practical learning. The incorporation of computer simulation and models provides enhancement and relevance to science learning.
Fostering high level of cognition
Also Mayer’s cognitive theory (2002), opined that students are more likely to engage in productive cognitive processing when corresponding words and pictures are presented at the same time. Simultaneous presentation increase the chances that corresponding words and pictures will be in working memory at the same time, thereby enabling the learner to construct mental connections between them. This cognitive processing results in deeper understanding as reflected in measures of problem solving transfer. Simultaneous presentation results in deeper learning than successive presentation, therefore students learn more deeply from multimedia presentations in which computer simulation is presented.
Overcome of misconception
There are many misconceptions involving all science fields. The large reason for many misconceptions is because of misrepresented information and diagrams in textbooks. This misinformation in textbooks can cause reinforcement of prior student misconceptions that they come into the classroom with. Not only do textbooks often convey wrong information to students, but a study by King (2010), carried out amongst 150 science teachers teaching earth science in England and Wales showed that teachers’ educational background in earth science was poor and that science textbooks were their key sources of information. Too often teachers are reinforcing their own misconceptions through the use of misrepresented texts and conveying their misconceptions to their students. As a panacea to these misconceptions, the use of computer simulation is important. When teachers know what their students think, they can implement instructional activities to challenge existing student’s ideas. Activities and questions can be planned in advance so teachers can target their students’ misconceptions. In line with Bakas and Mikropoulos (2003) children enter the class room with a wide range of misconceptions about plenary phenomena. The authors argue that conventional teaching method usually cannot overcome these difficulties, in part because of lack of appropriate teaching aids. Educational computer simulations support science teaching where abstract ideas and phenomena, impossible to be observed and experienced in other ways, are involved.
Many computer simulations are being used to enhance students’ conceptual development of abstract scientific phenomena. Micheal (2000), declare that computer simulation can afford learners numerous advantages. For example, computer simulations can: (1) provide the students with the opportunity to engage in activities that may otherwise be unattainable, (2) enhance academic performance and the learning achievement levels of students, and (3) be equally as effective as real-life hands on laboratory experiences.
Computer simulations are more engaging and seductive, and can teach complex topics with less need to simplify them. In a computer simulation model learners can easily and without effort visit places and view objects from different point of view, and can experiment by manipulating variables that cannot be manipulated in the real world. Gazit,E., Gal,E., Weiss,T. & Sachar, M. (2005) believes this and emphasized the notion that simulations are ideal for letting students explore things and construct their own knowledge.
Motivation of the learner
Computer simulation motivates students in the learning experience. Ramsden (1998), drew the conclusion that over the past few decades, young people have generally held unfavorable attitudes and beliefs towards science. It was recommended that to remediate the continuing decline in the number of students pursuing further study in science, teachers need to revise/refine their teaching and learning activities so that students’ motivation and engagement in science related activities will be increased. Barak, Ashkar and Doris (2011), sought to investigate not only the effect of animated movies on students’ learning outcomes, but on their motivation to learn. The authors investigated the use of web-based animated movies into the science curriculum of 4th and 5th grades students. The findings of the authors indicted that student who studies science with the use of animated movies developed higher motivation to learn science. These students developed higher motivation in term of: self –efficacy, interest and enjoyment, connection to daily life, and importance to their future, compared to the control group.
Extensive research has also shown a correlation between positive students’ perceptions and achievement. It has been reported that many studies of the relationship between motivation and learning achievement confirm that when students are more engaged in learning, they will more fully understand new knowledge and be more flexible in their use of it. By promoting intrinsic motivation, task involvement will increase and consequently, so will learning achievement.
Simulations in Support of Instruction
Computer simulation provides students with a very real experience and as such, they require a student’s absolute involvement and participation. Students are interested and more away of the topic and function as active participants (Hertel & mills, 2002). A constructive learning environment is created whereby students weave together interdependent elements and information to solve real world problems. Simulation thereby promotes a transfer of knowledge and helps with not only the education but also the application of a particular issue or concept. Ultimately students learn how to think critically in a complex situation (Brumfield, 2005). By placing emphasis on the learner as an active agent in the process of knowledge acquisition, simulations can support authentic inquiry practices that include formulating questions, hypothesis development, data collection and theory revision. By actively involving learners in exploring and discovering, simulation can be a powerful learning tool.
Simulation incorporates many factors to accommodate the different learning styles of the students. Computer simulation is a teaching technique that reproduces actual events and processes under test conditions. For example, when students attempt to understand processes of solar system; which cannot be done to scale for obvious reason, students must use simulations and interactive media to understand processes such as eclipse or moon phases. Simulation enables students to understand complex interactions of physical or social environment factors.
Here is a practical example of computer simulation on chemical bonding; the topic for the lesson is electrovalent or ionic bonding.
Electrovalent bond is characterized by transfer of electrons from metallic atoms to non-metallic atoms during a chemical reaction. The metallic atoms after donating their valence electrons become positively charged, while the non-metallic atoms become negatively charged when they gain electron. Elements with 1, 2 or 3 electrons in their outermost shell or energy levels can easily lose their valence electrons. In ionic bonding, after donating their valence electrons, their particles become negatively charged. These charged particles are known as ions.
Step 3: Examples of Electrovalent (Ionic) bonding
1. Formation of Sodium Chloride
Sodium has one electron in the outermost shell or energy level and chlorine has Seven electrons in the outermost shell or energy level, both of them achieve their octet configuration when they combine through ionic bonding.
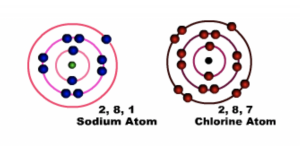
Fig 1 Before bonding
Sodium atom loses one electron in the outermost shell or energy level while Chlorine atom gains one electron. Both sodium and chlorine now have eight electrons in their outermost shell or energy level
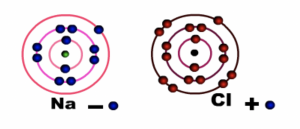
Fig 2: During bonding
Sodium ion (Na+) becomes positively charged and Chloride ion (Cl-) becomes negatively charged. Notice that sodium loses its outermost shell or energy level, while chlorine retains its outermost shell or energy level. The force of attraction between the two charged particles binds them together.
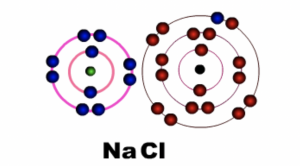
Fig 3: After bonding
The equation for the reaction can be written:
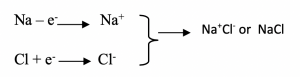
The electrical attraction between their opposite charges is the chemical bond that is found in sodium chloride. The chemical bond is called electrovalent or ionic bond.
Example 2: Formation of Calcium Oxide
Calcium has two electrons in the outermost shell or energy level while Oxygen has six electrons in the outermost shell or energy level.
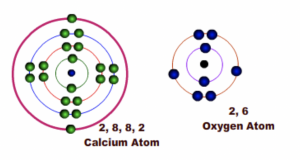
Fig 4: Before bonding
Calcium atom loses two electrons to Oxygen atom
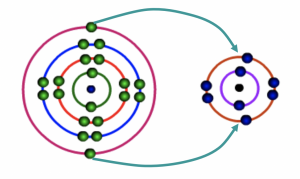
Fig 5: During bonding
Calcium becomes an ion (Ca2+) with two positive charges and Oxygen becomes Oxide ion (O2-) with two negative charges
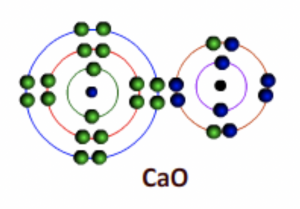
Fig 6: After bonding
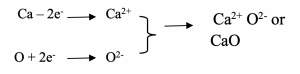
The equation for the reaction can be written as:
Challenges to the use of computer simulation and in teaching and learning chemistry
Educational reform includes successful designing and implementation of ICT in teaching and learning process, which is the key to success. It involves use of computers, software’s and other devices to store, convert and process, transmit and retrieve information. The act of integrating the use of ICT into teaching and learning especially in the developing countries like Nigeria is a complex process and one may encounter a number of difficulties
The major barriers were lack of genuine software, inadequate computer in the classroom, both teacher and students side to use ICT, lack of proper training experts/technical staff and poor administrative support. It tends to create anxiety for students given the unpredictable nature of the interaction.
Prospects of the use of computer simulation in teaching and learning
Technology integration has become popular in education and as teachers we are always looking for ways to effectively utilize technology tools in our classroom. As technology integration evolves in the classroom, we now have a wide variety of teaching tools available to help facilitate students learning. One of such tools is computer simulation.
- Whichever model is being used for providing simulation in teaching and learning, it promises a better performance of teachers in their actual classroom in future.
- In future when it is really going to be a tough challenge to meet training needs of the students’ in this computer age in real school environment, models of simulation will prove really helpful.
- Various projects concerning simulation for teaching and learning are under process and funded by many agencies of education.
- Like engineering and computer field simulation will be widely used in future due to cost effectiveness and ease of cost.
- It will help one to become better teacher in future
Conclusion
Computer simulation has been elaborately discussed, from the above discussion we can deduce that simulation is one of the instructional tools that aid the learners to achieve academic excellence as much as understanding and assimilation are concerned. The effect of simulation could be seen in the motivational factor it develops in learners. The learners are better motivated to learning through the use of simulation. Most abstract realities are made more concrete through these means of teaching and learning. Ultimately it is of importance to inculcate simulation in the learning curriculum so as to enhance the learning process and create room for advancement of knowledge in the 21st century. They should also create the awareness of the use of simulation to teachers of chemistry and other science teachers. It is the simulation of lessons that facilitate the interest of learners and eventual achievement of academic proficiency.
References:
Agbaje, R. O. &Alake, Ese. M., (2014) Students’ variable as predictor of secondary students’ Academic Achievement in science subjects, International Journal of Scientific and Research publications volume 4, issue 9, September 2014.
Aldrich, C. (2004). Simulation and the future of learning. An innovative (and perhaps revolutionary) approach to e- learning San Francisco, CA: Pfeiffer publisher.
Allesi, S.M., & Trollip, S. R.( 2001), Computer – Based Instruction: methods and Development(2nd ed), prentice Hall , NJ
Bakas, C. & Mikropoulos, T. A.,( 2003), Design of virtual environments for the comprehension of planetary phenomena based on students’ idea. International Journal of Science Education
Barak, M., Ashkar, T. & Dori, Y.J. (2011) Learning Science via Animated Movies: Its Effect on students’ Thinking and motivation. Computers and Education, 56(3), 839-846
Brumfield, R. (2005).September 6, Computer Simulation is ‘making history’ e school New online: where k-12 Education and Technology meet. Retrieved September 13, 2005 at http:/www. e schoolnews.com/ news pF show story. Cfm? Article ID=58632
Gazit, E., Gal, E., Weiss, T., & Sachar, M. (2005). Multi-user touch table as a tool for enhancing collaboration among austic children. The 3rd VR Symposium: Virtual Reality Associated Technologies and Rehabilitation Showcase. The University of Haifa,Haifa, Israel, 7-9 March 2005.
Giginna, L. I., Effect of Animation instructional strategy on students achievement, interest and retention in chemical bonding.
Goldsim, A.K.( 2011). Introduction to what is simulation. (Online)Available htt://www.goldsim.com/web/introduction/simulation-November12, 2012
Hertel, J.P. & Mills, B.J.(2002). Using simulation to promote learning I higher education. An introduction. Sterling, VA: stylus.
Institute of Simulation and Training, University of Florida, 2002
King, C. K.,( 2010). Simulation software vs expository text, A comparison of retention across two instructional tools in Reading. Research and Instruction, 28(2) 41-49
Kolomuc, A. & Tekin, S.,( 2011).Chemistry teachers misconceptions concerning concept of chemical reaction rate, Eurasian journal of physics and Chemistry Education, 3,( 2) 84-101.
Mayer, R. E. (2002). Multimedia learning, New York Cambridge University
Micheal, K. Y., (2000),’ Comparison of students’ product creativity using a computer simulation activity versus a hands-on activity in technology education,’ unpublished doctoral dissertation, Virginia polytechnic Institute and state University, VA
Federal Republic of Nigeria (FRN) in her National policy on education(2013), 6th ed. Abuja: NERDC Press
Ramsden, P. (1998) Managing the effective university. Higher Education Research and Development, 17(3); 347-370
Shu-Nu, C., Yau-Yuen, Y. & May, C. (2009) Ninth Graders’ learning Interests, life experiences and attitudes towards science and technology, Journal of Science Education and Technology.
Suleiman, M. A., (2011), Students integrated science achievement as predicator of later achievement in chemistry. A case study of selected schools in Zaria Metropolis, Scholar Research library, Achieves of Applied science Research 3(4). Retrieved from http:/ scholar research library.com/html
Tippler, M.N (2003). The objectives and creation of a course of simulation Michigan: Bostrom press lansing
West African Examination Council (WAEC) Chief Examiners reports, May/June 2010, 2011, 2012, 2013 and 2014.
Correspondence for: COMPUTER SIMULATION: AN INNOVATION IN TEACHING AND LEARNING OF CHEMISTRY IN SECONDARY SCHOOLS
Josephine C. Nnadi
Department of Art Education,
University of Nigeria Nsukka, Enugu State
josephinechinere555@Yahoo.Com

